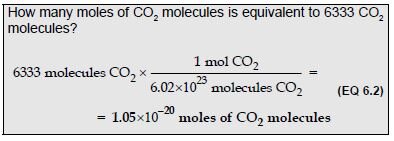Chemistry Problems: How to Solve Chemistry Problems On Your Own
/Do you know how to solve chemistry problems in these general chemistry topics? You can solve chemistry problems on your own, if you learn a few fundamental techniques.
dimensional analysis
moles
stoichiometry
gases
solutions
acids and bases
Chemistry problems can be hard to solve on your own
Understanding chemistry problems means understanding chemistry math problems.
Our chemistry professor has found over the years that the largest obstacle to student learning in chemistry have been those associated with math skills.
“Students have a difficult time applying what they have learned in their math classes to problems found in chemistry.”
To succeed in basic chemistry problems, you must be very comfortable with algebra and quantitative reasoning.
Don’t panic! There is hope for every student!
Take these 3 steps today!
You need one of these, because your professor probably wont let you use your phone calculator app.
How to solve chemistry math problems on your own: Your calculator is your friend!
Most schools have a check-out system for scientific calculators. Or you can find a used one on Amazon.
We have an entire free page on how to use your calculator to solve general chemistry problems.
Getting comfortable and quick with your calculator is essential for efficiently solving chemistry math problems.
2. Basic chemistry problems require specific vocabulary.
In Spanish class, you have to memorize the verb tenses. In football, you have to memorize the playbook.
In chemistry, you have to memorize words referring to measurement, nomenclature, and chemical reactions, among other essential vocabulary words. Words such as:
aqueous
barometer
coefficient
deci
element
functional group
gram
That’s quite an ABC!
Your chemistry text book has all the definitions you need. Make flash cards - they work!
Get it?
3. Moles and Molar Mass are all over general chemistry problems.
Moles refers to the number of molecules, number of atoms, number of ions, number of electrons, number of donuts, etc.
We would be really happy if we had 3 moles of chocolate ice cream (that’s three scoops!)
Avogadro’s number of items is called a mole of items.
Avogadro's number is 6.02x10 23.
You see? 1 mole of something is the same as Avogadro’s number of something.
Molar mass is an important concept when solving chemistry problems. The average atomic mass of an element (in grams) is the mass of 1 mole of atoms of that element.
Watch this video of our Joy Lab Chemistry Professor walking through a moles chemistry problem:
All your chemistry problems are solved in three easy steps!
Not really.
Chemistry is confusing, and has been for centuries. Just ask Dmitri Mendeleev.
For only $14, you can download your own guidebook to solving all first year chemistry problems!
This eBook is designed to help you through the obstacles of using math to solve chemistry problems.
9 chapters
complete chemistry problem solver guide
take what you learn here and apply to all general chemistry problems you will encounter in class
each chapter has chemistry practice problems and answers
bonus - the professor has a sense of humor!
Here at Joy Lab, we care about your chemistry problem solving success. We have a variety of resources to help.
Now, go do some chemistry!